How To Find Formal Charge In A Lewis Structure
Hey! Welcome to Master Organic Chemistry, just in instance you lot're a first time company.
In this blog mail service I explain how to calculate formal charge for molecules. Yet, you might discover my videos containing 10 solved examples of formal charge problems to be even more than useful. Just thought you should know!
Demand to figure out if an atom is negative, positive, or neutral? Here's the formula for figuring out the "formal charge" of an atom:
Formal charge = [# of valence electrons] – [electrons in lone pairs + 1/2 the number of bonding electrons]
This formula explicitly spells out the relationship betwixt the number of bonding electrons and their relationship to how many are formally "owned" by the atom.
For case, applying this to BH4 (top left corner in the image below) we go:
- The number of valence electrons for boron is 3.
- The number of non-bonded electrons is cipher.
- The full number of bonding electrons around boron is 8 (full octet). Ane half of this is four.
So formal charge = 3 – (0 + 4) = 3 – 4 = –ane
At that place is a slightly easier way to do this, even so.
Since a chemical bond has two electrons, the "number of bonding electrons divided by 2" is by definition equal to the number of bonds surrounding the atom. So we can instead apply this shortcut formula:
Formal Charge = [# of valence electrons on cantlet] – [non-bonded electrons + number of bonds].
Applying this again to BH4 (top left corner).
- The number of valence electrons for boron is 3.
- The number of not-bonded electrons is zero.
- The number of bonds around boron is 4.
And then formal charge = 3 – (0 + 4) = 3 – 4 = –i
The formal charge of B in BH4 is negative 1.
Let'due south apply information technology to :CH3 (ane to the right from BHfour)
- The number of valence electrons for carbon is 4
- The number of not-bonded electrons is two (it has a solitary pair)
- The number of bonds around carbon is 3.
Then formal charge = four – (2 +3) = 4 – v = –1
The formal charge of C in :CHthree is negative one.
Same formal charge as BH4!
Let's practice 1 last example. Let's do CH3 + (with no lone pairs on carbon). It'south the orange i on the bottom row.
- The number of valence electrons for carbon is 4
- The number of non-bonded electrons is zero
- The number of bonds around carbon is iii.
So formal charge = 4 – (0 +iii) = four – 3 = +ane
You tin can apply this formula to any atom you care to proper noun.
Here is a chart for some uncomplicated molecules forth the series B C N O . I hope beryllium and fluorine aren't likewise offended that I skipped them, only they're really non that interesting for the purposes of this tabular array.
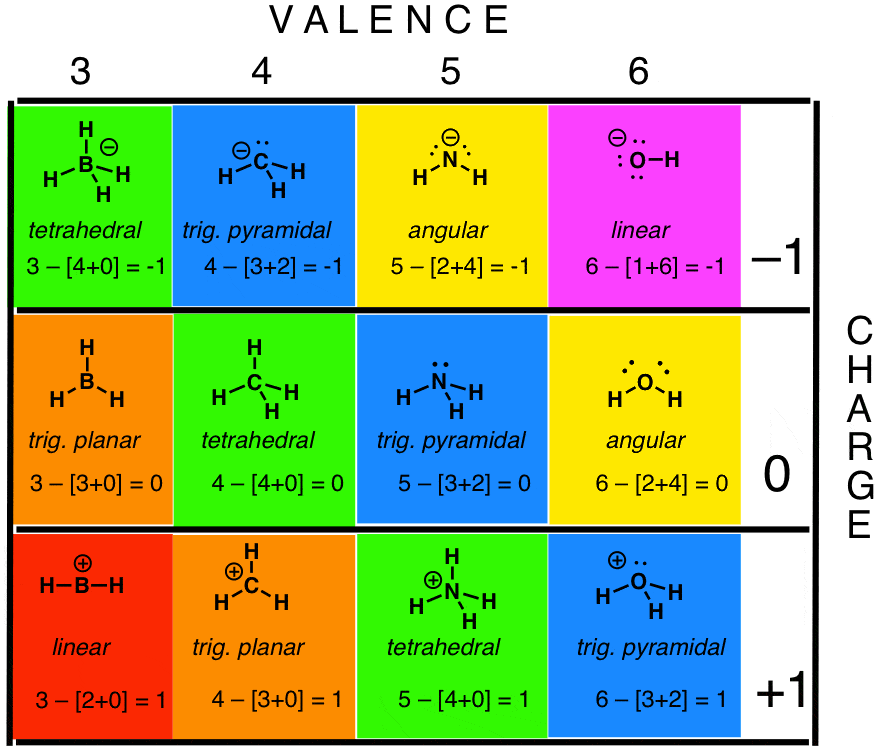
Annotation the interesting pattern in the geometries (highlighted in color): BHiv(–), CH4, and NH4(+) all have the same geometries, as do CH3(–), NH3, and OH3(+). Carbocation CHiii(+) has the aforementioned electronic configuration (and geometry) every bit neutral borane, BH3. The familiar bent structure of water, H2O, is shared by the amide anion, NH2(–). These shared geometries are one of the interesting consequences of valence crush electron pair repulsion theory (VSEPR – pronounced "vesper", but like "Favre" is pronounced "Farve".)
The formal charge formula also works for double and triple bonds:
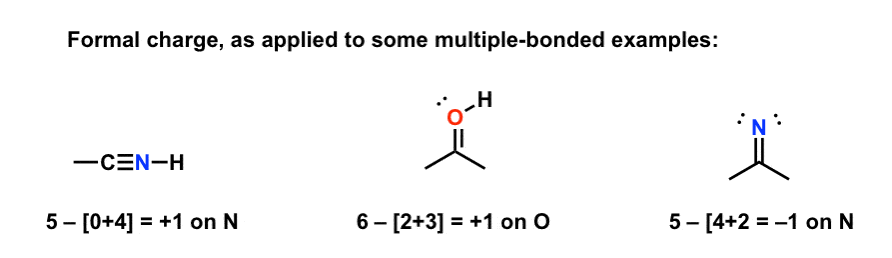
Here'southward a question. Alkanes, alkenes, and alkynes are neutral, since there are iv bonds and no unbonded electrons: 4 – [4+0] = 0. For what other values of [bonds + nonbonded electrons] will you also get a value of null, and what might these structures look similar? (Y'all'll meet some of these structures later in the course).
1 terminal question – why do you lot think this is called "formal accuse"?
Call up virtually what the formal charge of BF4 would exist. Negative charge on the boron. What's the most electronegative element here? Fluoride, of grade, with an electronegativity of four.0, with boron clocking in at 2.0. Where do you think that negative charge really resides?
Well, information technology own't on boron. Information technology's really spread out through the more than electronegative fluoride ions, which become more than electron-rich. And then although the "formal" accost of the negative charge is on boron, the electron density is actually spread out over the fluorides. In other words, in this case the formal accuse bears no resemblance to reality.
Another reminder – 10 videos with solved examples of formal charge problems, right hither (look at the very top of the page)
Source: https://www.masterorganicchemistry.com/2010/09/24/how-to-calculate-formal-charge/
Posted by: andrewswitis1960.blogspot.com

0 Response to "How To Find Formal Charge In A Lewis Structure"
Post a Comment